The kcat/km ratio is a measure of the catalytic efficiency of an enzyme. It tells us how fast an enzyme can convert a substrate into a product under different conditions. In this post, we will explain what the kcat/km ratio means, how it is calculated, and what factors affect it.
kcat and km; introduction
Kcat is the turnover number of an enzyme. It is the maximum number of substrate molecules that one enzyme molecule can convert into a product per unit of time when the enzyme is fully saturated with substrate. It is also called the limiting rate constant because it represents the upper limit of the reaction rate that the enzyme can achieve.
Kcat is calculated by dividing the maximum reaction rate (Vmax) by the total enzyme concentration ([E]T):
$$k_{cat} = \frac{V_{max}}{[E]_T}$$
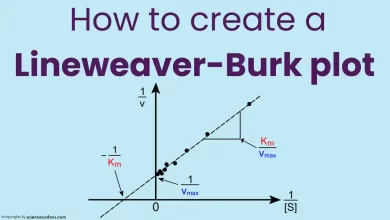
Km is the Michaelis constant of an enzyme. It is the substrate concentration at which the reaction rate is half of the maximum rate (Vmax/2). It is also a measure of the enzyme’s affinity for the substrate. The lower the km, the higher the affinity, and the less substrate is needed to reach half of the maximum rate.
Km is calculated by dividing the sum of the rate constants for the dissociation of the enzyme-substrate complex (k-1) and the formation of the product (k2) by the rate constant for the formation of the enzyme-substrate complex (k1):
$$k_m = \frac{k_{-1} + k_2}{k_1}$$
What is the kcat/km ratio, and why is it important?
kcat/km is the ratio of the turnover number and the Michaelis constant. It is also called the specificity constant because it reflects how specific the enzyme is for the substrate. The higher the kcat/km, the more efficiently the enzyme converts the substrate into a product.
kcat/km is calculated by dividing the turnover number by the Michaelis constant:
$$\frac{k_{cat}}{k_m} = \frac{V_{max}}{[E]_T} \cdot \frac{k_1}{k_{-1} + k_2}$$
kcat/km can also be interpreted as the second-order rate constant for the reaction between the enzyme and the substrate when the substrate concentration is very low and much lower than the km. In this case, the reaction rate is proportional to the product of the enzyme concentration and the substrate concentration, and kcat/km is the proportionality constant:
$$v = \frac{k_{cat}}{k_m} [E]_T [S]$$
What factors affect kcat/km?
The kcat/km ratio depends on several factors, such as the structure and properties of the enzyme and the substrate, the pH and temperature of the environment, and the presence of inhibitors or activators. Some examples of how these factors can affect kcat/km are:
-Structure of the enzyme and the substrate
The structure and properties of the enzyme and the substrate determine how well they fit together and how easily they react. For example, if the enzyme has a flexible active site that can adjust to the shape of the substrate, it may have a higher kcat/km than an enzyme with a rigid active site. Similarly, if the substrate has a high reactivity, it may have a higher kcat/km than a substrate with a low reactivity.
-The pH and temperature
The pH and temperature of the environment affect the stability and activity of the enzyme and the substrate. For example, suppose the pH or temperature is too high or too low. In that case, it may denature the enzyme or the substrate, reducing their affinity and reactivity and lowering the Kcat/km. On the other hand, if the pH or temperature is optimal for the enzyme or the substrate, it may enhance their affinity and reactivity, increasing the Kcat/km.
-Presence of inhibitors or activators
The presence of inhibitors or activators can modulate the activity and efficiency of the enzyme. For example, suppose a competitive inhibitor binds to the same active site as the substrate. In that case, it may reduce the availability of the enzyme for the substrate and lower the kcat/km. On the other hand, if an allosteric activator binds to a different site on the enzyme and changes its conformation, it may increase the affinity and reactivity of the enzyme for the substrate and improve the Kcat/km.
Example
Let’s look at an example of how to calculate and compare the kcat/km ratios of two enzymes, A and B, that catalyze the same reaction:
$$S \xrightarrow{enzyme} P$$
The following table shows the kinetic parameters of the two enzymes:
| Enzyme | Vmax (mol/min) | [E]T (mol/L) | km (mol/L) |
|---|---|---|---|
| A | 0.1 | 0.001 | 0.01 |
| B | 0.05 | 0.0005 | 0.005 |
To calculate the kcat/km ratio of enzyme A, we use the formula:
$$\frac{k_{cat}}{k_m} = \frac{V_{max}}{[E]_T} \cdot \frac{1}{k_m}$$
Plugging in the values from the table, we get:
$$\frac{k_{cat}}{k_m} = \frac{0.1}{0.001} \cdot \frac{1}{0.01} = 1000 \ L/mol \cdot min$$
To calculate the kcat/km ratio of enzyme B, we use the same formula and plug in the values from the table:
$$\frac{k_{cat}}{k_m} = \frac{0.05}{0.0005} \cdot \frac{1}{0.005} = 2000 \ L/mol \cdot min$$
Comparing the kcat/km ratios of the two enzymes, we can see that enzyme B has a higher catalytic efficiency than enzyme A, even though it has a lower maximum reaction rate. This means that enzyme B can convert more substrate into product per unit time and unit enzyme concentration than enzyme A, especially when the substrate concentration is low. Enzyme B is also more specific for the substrate than enzyme A, because it has a lower km, which means it has a higher affinity for the substrate.
More information:
- https://www.pearson.com/channels/biochemistry/learn/jason/enzymes-and-enzyme-kinetics/specificity-constant (RECOMMENDED)
- aklectures.com. https://aklectures.com/lecture/fundamentals-enzymes/catalytic-efficiency-of-enzymes-kcat-km.
- What is the meaning behind Kcat / Km? – Biology Stack Exchange. https://biology.stackexchange.com/questions/53483/what-is-the-meaning-behind-kcat-km.
- Structural Biochemistry/Enzyme/Kcat/Km – Wikibooks. https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Kcat/Km.
- https://www.biorxiv.org/content/10.1101/2021.12.09.471995v1.full
- sciencedirect.com
- https://aclanthology.org/P19-3017.pdf