Blood coagulation, also called blood clotting, is a crucial process that prevents excessive bleeding when a blood vessel is injured. Hemostasis is essential for maintaining the integrity of blood vessels and preventing blood loss. In this article, we will explore the process of blood clot formation, with a specific focus on the roles and interactions of platelets and coagulation factors.
The blood coagulation steps
The process of blood coagulation occurs in multiple stages.
1) Vasoconstriction is a protective response that occurs when a blood vessel is injured. This process involves the constriction of blood vessels, which reduces blood flow and minimizes bleeding. Local factors released at the site of injury cause the contraction of smooth muscle cells in blood vessel walls, leading to vasoconstriction.
2) Platelet Aggregation: When vasoconstriction is insufficient to halt bleeding, platelet aggregation takes place, resulting in the formation of a platelet plug. Platelets, derived from megakaryocytes, play a crucial role in this process. The injured tissue releases various factors, including the von Willebrand factor, that activate platelets. When platelets encounter a damaged vessel wall, they change shape and become activated. This activation leads to increased platelet collision and aggregation. Platelets contain ADP and thromboxane A2, which enhance their activation and ability to clump together. The accumulation of platelets forms a plug at the site of injury, aiding in the closure of the damaged blood vessel.
3) Blood Coagulation: The process of blood coagulation begins after platelet aggregation. Platelet aggregation triggers the activation of coagulation factors, leading to the formation of a blood clot. The clot, made up of fibrin strands, incorporates the platelet plug. This transformation of liquid blood into a gel-like substance effectively seals off the injured area and promotes clotting.
4) Tissue Repair: Once hemostasis is achieved, damaged tissue requires time to undergo the repair process. Platelet-derived factors play a crucial role in the repair process. Blood coagulation is a vital component of hemostasis and aids in the formation of blood clots, which are critical for tissue repair. Blood coagulation is a component of the broader function of hemostasis.
Summary of the above steps
- The initial response to injury involves damage to the endothelial cells, which leads to vasoconstriction and partial closure of the affected blood vessel.
- Platelet aggregation leads to the formation of a platelet plug.
- Blood coagulation reactions are then initiated, resulting in the formation of fibrin strands and the subsequent formation of a blood clot.
- The blood clot remains until the damaged tissue is repaired.
- Once tissue repair is complete, the clot is gradually dissolved through specific mechanisms.
- Understanding the complex process of blood coagulation is crucial for comprehending the mechanisms that maintain hemostasis and prevent excessive bleeding.
Platelets and Blood Coagulation
In normal circumstances, blood flows smoothly through the vessels due to the unique characteristics of the vessel walls. Platelets, inactive in their resting state, can easily pass through the endothelial cells lining vessel walls.
Endothelial cells produce prostacyclins, which are potent factors that prevent platelet aggregation and adhesion. As long as the endothelial layer of the blood vessels remains healthy, platelets remain inactive.
When the endothelial layer is damaged, whether through microscopic injuries or larger ruptures, platelet adhesion is activated. Activated platelets release factors that attract more platelets, resulting in a cascade of platelet activation and the formation of a platelet plug.
Several factors contribute to platelet activation, such as:
- Microscopic injuries to the endothelial layer can occur.
- Larger damages to the vessel wall expose collagen.
- Positive feedback occurs among platelets.
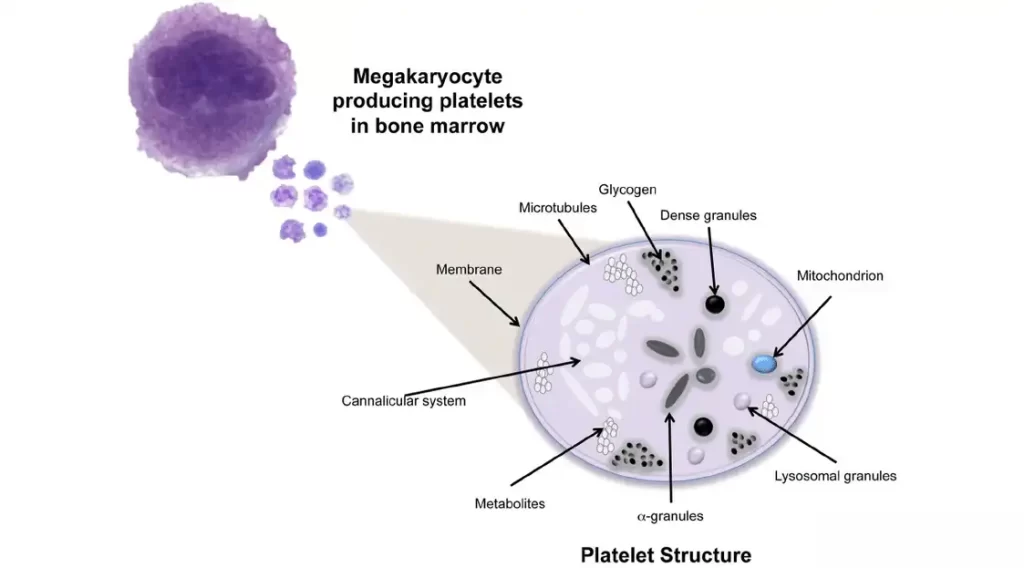
Platelet Structure
Platelets contain proteins, including actin, myosin, and thrombosthenin, in their cytoplasm. Actin and myosin play a role in platelet contraction, which aids in maintaining the integrity of the blood clot after coagulation.
The contraction of the blood clot leads to the release of plasma, which forms serum. Coagulation factors, including activating factors, can be diverse, either internal or external. Platelets play a role in stabilizing blood clots by using their contractile elements, actin and myosin.
Platelets have a well-developed network of endoplasmic reticulum and Golgi apparatus in their cytoplasm, which allows for the production of enzymes that are released when the platelets are activated. They also serve as a calcium source and contain mitochondria that produce ATP and ADP, which are essential for platelet function. Platelets also contain powerful enzymatic systems.
One important protein released by platelets is the fibrin stabilizing factor, which strengthens fibrin strands. The contraction process starts with the formation of fibrin strands. Platelets play a role in clot formation and blood coagulation by producing and releasing the fibrin-stabilizing factor, which helps make the clot more durable. Consequently, platelet plugs and blood clots fill the damaged area. Over time, the damaged area undergoes repair with the help of growth factors from platelets. Platelet-derived growth factor (PDGF) plays a crucial role in the healing of damaged areas. Collagen and von Willebrand factor are both factors that activate platelets.
Summary of Platelet Function in Blood Coagulation

Under normal conditions, certain factors keep platelets inactive, allowing them to circulate in the bloodstream freely. Prostacyclins and nitric oxide (NO) are continuously released from endothelial cells. As long as the endothelial cells lining the vessel walls remain intact, the production of prostacyclins and NO prevents platelet activation.
When the endothelial layer is damaged, either visibly or due to prolonged factors such as high blood pressure or other influences on endothelial cells, it can disrupt the release of prostacyclins and NO. This disruption can trigger platelet activity, leading to platelet aggregation and the formation of blood clots or thrombi. The intact endothelial layer acts as a barrier, preventing direct interaction between platelets and collagen in the vessel wall. This interaction is necessary for platelet activation and the formation of blood clots.
In cases of apparent endothelial damage or microscopic conditions where endothelial cells are affected by long-term factors like fatty plaques or other vascular damages, endothelial injury can occur, initiating the process of blood coagulation.
Coagulation and Fibrinolysis of Blood
Coagulation and fibrinolysis are continuous processes that are essential for the proper functioning of blood as a tissue. Blood must flow smoothly and be able to clot when there is bleeding in various areas.
Blood coagulation involves multiple factors. Factor I, also known as fibrinogen, is responsible for producing fibrin strands. Platelets release the fibrin stabilizing factor to ensure the stability of these strands. Other factors, both internal and external, work together to activate and facilitate the blood clotting process.
Deficiencies in the production or levels of these factors can result in bleeding disorders and diseases like hemophilia type A and B, or other conditions that disrupt the blood clotting process. The activation or inactivation of these factors is tightly regulated by cellular mechanisms that maintain a delicate balance between procoagulant and anticoagulant factors, ensuring proper blood flow.
Pathways of Blood Coagulation
During the initiation of coagulation, two pathways are involved: the internal and external pathways. Although these pathways have distinct names and are explained separately for clarity, they share many common elements. It is not scientifically significant to strictly differentiate between them.
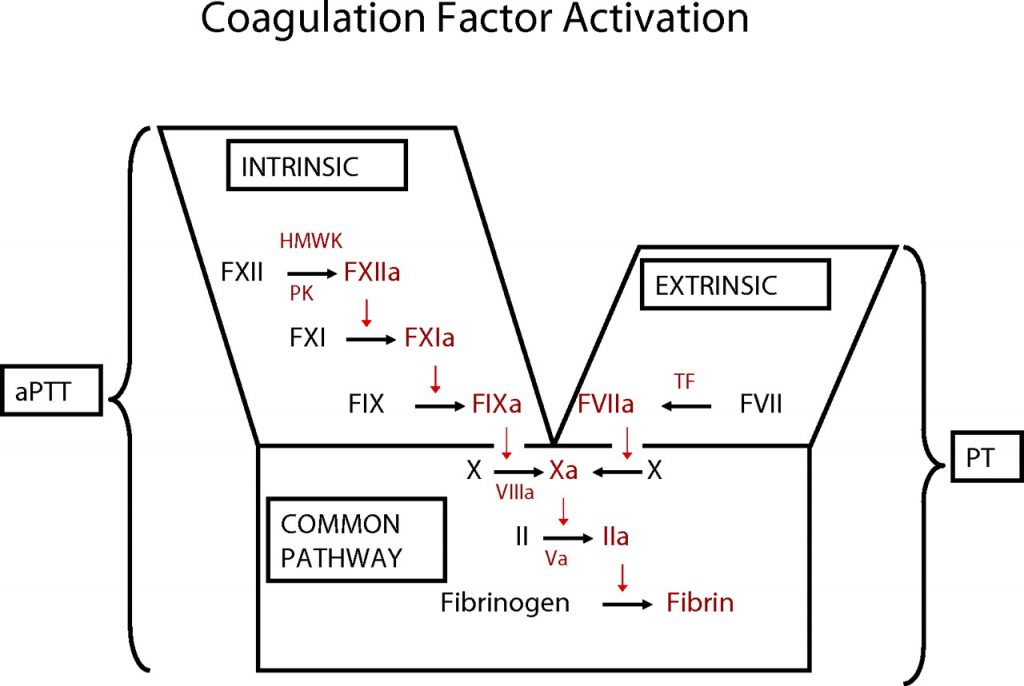
The common point in both pathways is the production of fibrin strands, which are stabilized by the fibrin stabilizing factor secreted by platelets. Fibrinogen is activated and forms fibrin. Thrombin, under the influence of specific factors, converts fibrinogen into fibrin. Ultimately, polymerized fibrin strands form a clot, trapping platelets and other blood components, which initiates the blood coagulation process.

Coagulation of Blood Common Pathway
The diagram above illustrates that factor 10 is activated by factor 5, the presence of calcium, and platelet involvement. Activated factor X triggers the conversion of prothrombin into thrombin. Thrombin facilitates the conversion of fibrinogen into fibrin. This stage is the final step in the blood coagulation process. Factors that activate factor 10 can trigger blood coagulation.
Fibrinolysis
Fibrinolysis is the natural process of dissolving blood clots (fibrin) within the body. When a clot forms, it can either be repaired by fibroblasts and healing factors in damaged tissue or eliminated through fibrinolytic reactions.
The process of clot dissolution, known as fibrinolysis, starts with the breakdown of fibrin strands. Fibrin can be degraded by the enzyme plasmin. Plasminogen is converted into plasmin through the activation process facilitated by activators. Plasmin not only digests fibrin strands but also functions as an anticoagulant by deactivating fibrinogen. It can also deactivate blood coagulation factors II, V, VIII, and XII.
In clinical settings, clot destruction or dissolution can be achieved using protease drugs. Urokinase, streptokinase, and tissue plasminogen activator (TPA) are medications that can dissolve blood clots. Tissue plasminogen activator (TPA) can be produced by damaged endothelial cells or generated in the laboratory for therapeutic use by physicians in cases where blood clots or thrombi have formed.
The anticoagulant system is a systemic mechanism that prevents blood clotting in normal circumstances. The release of factors from endothelial cells is the primary mechanism for preventing blood coagulation. Endothelial cells continuously produce these factors under normal conditions.
If the process of preventing blood coagulation is disrupted, clots may form. The fibrinolysis mechanism is responsible for dissolving and removing blood clots from the bloodstream.
The regulation of coagulation requires a delicate balance between procoagulant and anticoagulant factors. Both procoagulant and anticoagulant factors are necessary for proper blood clotting and clot dissolution.
The endothelial layer and blood cells are essential in this process. Smooth muscle in the vessel wall, the glycocalyx layer of endothelial cells, thrombomodulin, and tissue plasminogen activators released from the endothelial layer are all crucial factors. Prostaglandins and nitric oxide (NO) are important regulators of coagulation.
When fibrin strands form, they create a balance between coagulation and anticoagulant factors in the blood by consuming coagulation factors and other factors.
Antithrombin III
Antithrombin III is a crucial substance in this process. Heparin increases the effectiveness of antithrombin III. Antithrombin III is a protein that inhibits thrombin and activates the body’s anticoagulant system. The protein C system is activated by thrombin and ultimately breaks down fibrin strands using potent proteolytic enzymes in the fibrinolytic system.
Antithrombin III is a serine protease inhibitor that plays a crucial role in inactivating coagulation factors II, IX, X, XI, and XII.
In the presence of heparin, the activity of antithrombin III can increase by up to a thousandfold. Heparin is a polysaccharide produced primarily by mast cells in organs such as the lungs and liver. Therefore, the combination of heparin and antithrombin III always ensures that blood flow creates conditions that prevent blood coagulation.
Protein C
thrombomodulin and the protein C system are anticoagulant systems produced by healthy endothelial cells. Thrombomodulin acts as an inhibitor of blood clotting and deactivates thrombin. It also activates protein C, which is essential for breaking down and degrading coagulation factors 5 and 8.
Here is a summary of the conditions and disorders associated with blood clotting.
If coagulation factors weaken, it can lead to hypo-coagulation and spontaneous bleeding, also known as hemorrhage.
In situations where coagulation factors are heightened, excessive blood coagulation occurs, leading to the formation of blood clots (thrombus). When a blood clot becomes dislodged from the vessel wall and starts to move, it is called an embolus.
Conditions that can lead to reduced blood coagulation and subsequent bleeding include thrombocytopenia (a decrease in platelets), deficiencies in coagulation factors (such as hemophilia A and B), and vitamin K deficiency (which hinders the synthesis of factors 2, 7, 9, and 10).
Hypercoagulation can lead to thromboembolic diseases, which can cause blockages in blood vessels, coronary artery diseases, and systemic arterial occlusion. In cases of hypercoagulation, excessive consumption of coagulation factors leads to a deficiency of these factors in the bloodstream. A deficiency in this area can result in disseminated intravascular coagulation (DIC). In disseminated intravascular coagulation (DIC), blood flow is obstructed at multiple points, leading to both bleeding and a lack of coagulation factors. Disseminated intravascular coagulation (DIC) can lead to excessive bleeding and ultimately result in hypovolemic shock, which is characterized by low blood pressure and the development of a severe, life-threatening condition.