Kcat is a parameter that measures the catalytic efficiency of an enzyme. It indicates how many substrate molecules an enzyme can convert into a product per unit of time when fully saturated with substrates. Kcat is also known as the turnover number or the catalytic constant.
How to calculate Kcat
To calculate Kcat, we need to know two other parameters: Vmax and [E]. Vmax is the maximum velocity of the enzyme-catalyzed reaction, which is reached when the enzyme is saturated with substrates. [E] is the total concentration of active enzyme.
What is the formula for Vmax and kcat?
$$Kcat = {{Vmax} \over [Et]}$$
Kcat units: S-1
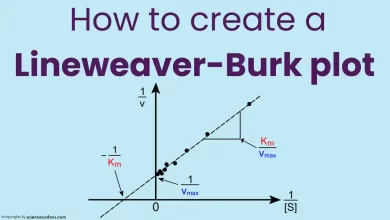
Vmax can be determined experimentally by plotting the initial velocity (v) of the reaction against the substrate concentration ([S]) and finding the asymptotic value on the y-axis. Alternatively, Vmax can be calculated using the Michaelis-Menten equation, which describes the relationship between v, [S], Vmax, and Km. Km is another parameter that measures the enzyme’s affinity for its substrate. The Michaelis-Menten equation is:
$$v = {{Vmax [S]} \over Km+[S]}$$
By rearranging the equation, we can solve for Vmax:
$$Vmax = {{v(Km+[S])} \over [S]}$$
Once we have Vmax, we can plug it into the Kcat equation and obtain the value of Kcat.
Why Kcat is important
Kcat is essential because it reflects the intrinsic ability of the enzyme to catalyze the reaction. It is independent of the enzyme concentration, unlike Vmax, which depends on how much enzyme is used in the experiment. Therefore, Kcat is a more reliable and comparable measure of enzyme performance.
Kcat can also be used to calculate the catalytic efficiency of the enzyme, which is defined as the ratio of Kcat to Km:
$$Catalytic efficiency = {{Kcat} \over Km}$$
The catalytic efficiency indicates how well the enzyme utilizes its substrate at low concentrations. A higher catalytic efficiency means the enzyme can achieve a high reaction rate with a low substrate concentration, which is desirable for most biological processes.
Examples of Kcat values
Different enzymes have different Kcat values depending on their structure, function, and substrate specificity. Some examples of Kcat values for various enzymes are:
- Carbonic anhydrase: 106 s-1
- Acetylcholinesterase: 104 s-1
- Hexokinase: 102 s-1
- DNA polymerase: 10 s-1
These values show that carbonic anhydrase is the fastest enzyme, capable of converting one million carbon dioxide molecules into bicarbonate per second. On the other hand, DNA polymerase is the slowest enzyme, converting only 10 molecules of nucleotides into DNA per second. These differences reflect these enzymes’ different roles and requirements in the cell.
Reference:
https://www.nature.com/articles/s41929-022-00798-z