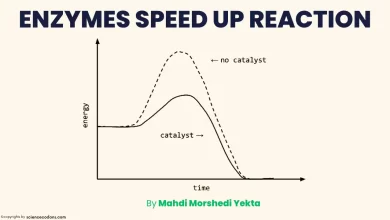
Any substance that can reduce the rate of chemical reactions is called an inhibitor. Many inhibitors have medicinal applications. Enzyme inhibitors are divided into different categories, and today, we will compare Competitive and Noncompetitive inhibition.
Competitive inhibitors of enzymes
Competitive inhibition occurs when a molecule that resembles the normal substrate of an enzyme competes with the substrate for binding to the enzyme’s active site. The active site is the region on the enzyme where the substrate binds, and the reaction takes place. By occupying the active site, the competitive inhibitor prevents the substrate from binding and reduces the reaction rate.
The effect of competitive inhibition depends on the concentration of the substrate and the inhibitor. If the substrate concentration is high, the substrate can outcompete the inhibitor and bind to the enzyme more frequently, restoring the reaction rate. Therefore, competitive inhibition can be overcome by increasing the substrate concentration. However, if the inhibitor concentration is high, the inhibitor will have a greater chance of binding to the enzyme and reducing the reaction rate. Therefore, competitive inhibition can be enhanced by increasing the inhibitor concentration.
noncompetitive inhibitors of enzymes examples
- Nifedipine: Inhibits the activity of the CYP2C9 enzyme.
- Tranylcypromine: Inhibits the activity of monoamine oxidase (MAO) enzyme.
- Phenethyl isothiocyanate: Inhibits the activity of the myrosinase enzyme.
- 6-Hydroxyflavone: Inhibits the activity of the protein kinase C (PKC) enzyme.
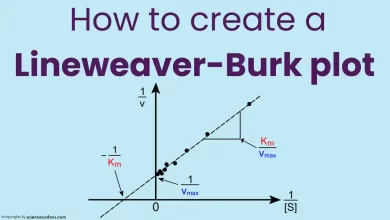
What happens to Km and Vmax in competitive inhibition?
The kinetic parameters of an enzyme, such as the maximum velocity (Vmax) and the Michaelis-Menten constant (Km), can be used to quantify the effect of competitive inhibition. The Vmax is the maximum reaction rate when the enzyme is saturated with the substrate. The Km is the substrate concentration at which the reaction rate is half the Vmax. The lower the Km, the higher the enzyme’s affinity for the substrate.
In competitive inhibition, the Vmax of the enzyme does not change because the inhibitor does not affect the enzyme’s catalytic ability. However, the Km of the enzyme increases because the inhibitor reduces the enzyme’s affinity for the substrate. This means that a higher substrate concentration is needed to achieve the same reaction rate as in the absence of the inhibitor.
Noncompetitive inhibitors of enzymes
Noncompetitive inhibition (allosteric inhibition) occurs when a molecule that does not resemble the normal substrate of an enzyme binds to a site other than the enzyme’s active site. This site is called an allosteric site, and it can influence the shape and function of the enzyme. By binding to the allosteric site, the noncompetitive inhibitor changes the conformation of the enzyme and makes it unable to catalyze the reaction.
The effect of noncompetitive inhibition does not depend on the concentration of the substrate because the inhibitor does not compete with the substrate for binding to the enzyme.
What happens to Km and Vmax in noncompetitive inhibition?
The kinetic parameters of an enzyme, such as the Vmax and the Km, can be used to quantify the effect of noncompetitive inhibition. The Vmax of the enzyme decreases because the inhibitor lowers the amount of active enzyme. However, the Km of the enzyme does not change because the inhibitor does not affect the enzyme’s affinity for the substrate
competitive inhibitors of enzyme examples.
- Methotrexate: a drug that is used to treat cancer and rheumatoid arthritis. Inhibits the activity of dihydrofolate reductase enzyme.
- Sildenafil: Inhibits the activity of phosphodiesterase type 5 (PDE5) enzyme.
- Malonic acid: Inhibits the activity of succinate dehydrogenase enzyme.
- Sulfanilamide: Inhibits the activity of dihydropteroate synthase enzyme.
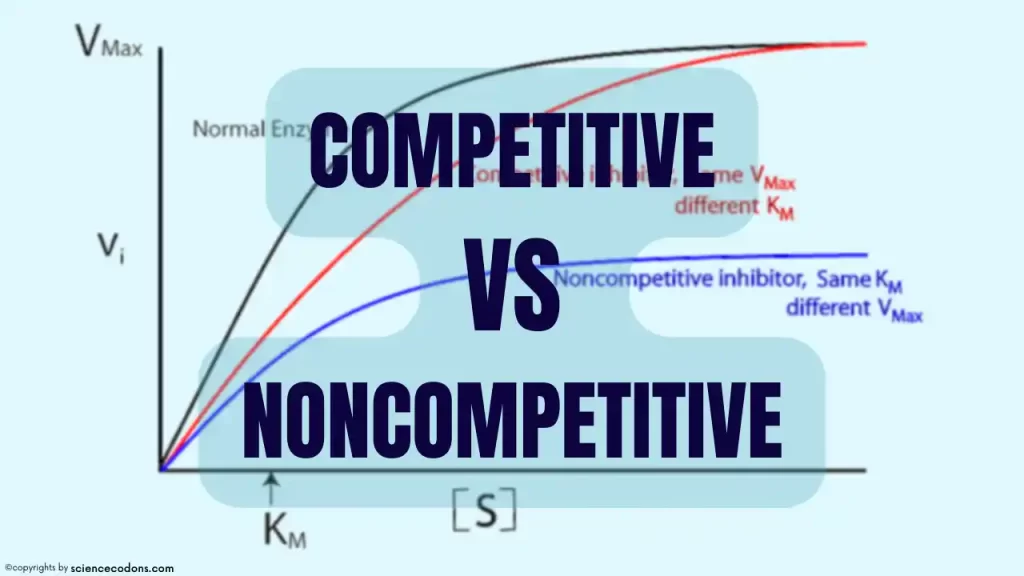
Competitive vs. Noncompetitive inhibition graph
In the competitive inhibition graph: the maximum reaction rate (Vmax) is unchanged, but the substrate concentration needed to reach half of Vmax (Km) is increased.
In the noncompetitive inhibition graph: the inhibitor reduces the number of active enzyme molecules available for catalysis. As a result, the maximum reaction rate (Vmax) is decreased, but the Km is unchanged.
Summary
Competitive and noncompetitive inhibition are two types of enzyme inhibition that have different mechanisms and effects on enzyme activity and kinetics. Competitive inhibition occurs when a molecule similar to the substrate competes with the substrate for binding to the enzyme’s active site. Noncompetitive inhibition occurs when a molecule, which does not resemble the substrate, binds to an allosteric site of the enzyme and changes its conformation. Increasing substrate concentration can overcome competitive inhibition, whereas noncompetitive inhibition cannot. Competitive inhibition increases the Km of the enzyme, whereas noncompetitive inhibition decreases the Vmax of the enzyme.
| Type of Inhibition | Binding Site | Effect on Vmax | Effect on Km | Example |
|---|---|---|---|---|
| Competitive | Active site | Decreases | Unchanged | Malonate |
| Noncompetitive | Allosteric site | Decreases | Decreases | Iodoacetamide |
Reference: