Glycoproteins are proteins to which sugar chains are attached via covalent bonds. Almost all plasma proteins, except for albumin, are glycoproteins. Many membrane proteins, blood group antigens, and some hormones are also classified as glycoproteins.
What is glycoprotein and its function?
Glycoproteins are proteins to which one or more sugar chains are attached. The major portion of their structure is composed of protein, with only a small part being formed by sugar chains. Although over 200 monosaccharides have been identified in nature, only eight monosaccharides are primarily found in glycoproteins. These intricate macromolecules play crucial roles in various biological processes. Here’s a concise table summarizing their functions along with examples:
| Function | Description | Examples |
|---|---|---|
| Biological Recognition | Glycoproteins aid in cell recognition and processes like fertilization. | Sperm-egg recognition, receptor proteins |
| Immune System | Antigens for immune response; blood type determination | Red blood cell surface antigens |
| Structural Roles | Contribute to tissue integrity | Fibrillins |
| Hormones and Signaling | Function as hormones or participate in signaling pathways | Tumor necrosis factor |
| Integral Membrane Proteins | Key roles in cell communication and transport | Membrane glycoproteins |
Glycoprotein Classification
Classification Based on the type of linkage between the sugar chain and the protein, glycoproteins can be divided into three groups:
- O-Glycoproteins: In these glycoproteins, the sugar chain is attached to the hydroxyl group present in the side chain of serine or threonine amino acids.
- N-Glycoproteins: Here, the sugar chain is linked to the nitrogen of the asparagine side chain in the protein.
- Phosphorylethanolamine-linked Glycoproteins: The sugar chain is connected to the C-terminal end of the protein via phosphorylethanolamine linked to glucosamine.
O-Glycoproteins
In O-linked glycosylation, sugars are attached to oxygen atoms, typically on serine or threonine residues. They can also occur on tyrosine or non-canonical amino acids like hydroxylysine and hydroxyproline. O-Glycoproteins Glycoproteins with glycosidic bonds can be further divided into four smaller groups:
- N-Acetyl Galactosamine-Serine (or Threonine) Linked Glycoproteins: Examples include mucins.
- Galactose-Galactose-Glucose-Serine Linked Glycoproteins: Examples include proteoglycans.
- Galactose-Hydroxylysine Linked Glycoproteins: Examples include collagen.
- N-Acetyl Glucosamine-Serine (or Threonine) Linked Glycoproteins: Examples include nuclear proteins.
Biological Significance
Biological Significance Mucins are glycoproteins found in secretions of the digestive, respiratory, and reproductive systems and in various cell membranes. There are two types of mucins: secretory and membrane-bound.
Secretory mucins are found in the secretions of the digestive and respiratory systems and contribute to mucus formation. The mucus is composed of 94% water and 5% mucin glycoproteins. The mucus serves as a protective physical barrier on epithelial surfaces. Mucins also play a role in cell-to-cell interactions and may contain some surface antigens or absorb them. Membrane-bound mucins are involved in cell-cell interactions. Mucins attached to the membrane contribute to forming some membrane surface antigens. In certain types of cancer, cell surfaces are often covered by an abundance of mucin. These proteins have two main characteristics: their carbohydrate content exceeds 50%, and their structure contains repetitive amino acid sequences rich in serine, threonine, and proline.
Synthesis
Synthesis The protein portion of O-glycoproteins is synthesized on ribosomes attached to the rough endoplasmic reticulum. The protein segment is then packaged in vesicles and transported to the Golgi apparatus. In the Golgi apparatus, enzymes called glycoprotein glucosyltransferases remove sugar molecules from nucleotide-bound sugars and transfer them to protein nuclei. The final sugar units are added to the trans side of the Golgi apparatus to form glycoproteins, which are then secreted from the cell.
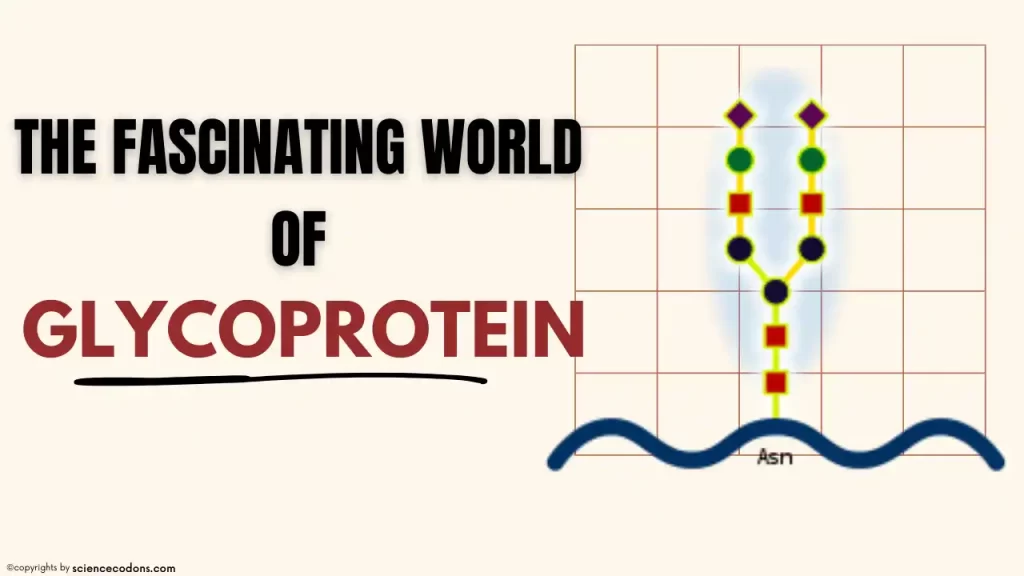
N-Linked Glycoproteins
Structure
In N-linked glycosylation, sugars are attached to nitrogen atoms, typically on the amide side-chain of asparagine (Asn-x-Ser/Thr motifs) in glycoproteins. N-linked glycoproteins with glycosidic bonds have a common core structure, as depicted in the diagram. The side branches that attach to this central core differ among N-linked glycoproteins. Let’s delve into this intriguing topic:
Common Core Structure
N-linked glycoproteins share a common core structure: an oligosaccharide chain (glycan) attached to the asparagine residue within the protein. This core structure is often referred to as the N-glycan.
Variability in Side Branches
The specific side branches (or antennae) that extend from the N-glycan can vary significantly. These side branches contain different sugar monomers (such as glucose, mannose, galactose, fucose, and sialic acid). The arrangement and composition of these sugars determine the overall glycan structure.
Functional Implications
- The diversity of side branches influences various aspects of glycoprotein function:
- Cell-Cell Recognition: Different glycan structures allow cells to recognize each other during processes like immune responses and tissue development.
- Stability and Folding: Glycans contribute to protein stability and proper folding.
- Receptor Binding: Glycans on cell surface receptors play a role in ligand binding.
- Modulation of Protein Properties: Glycans can affect protein solubility, half-life, and interactions.
Glycosylation Sites:
- Not all asparagine residues within a protein become glycosylated.
- The specific amino acid sequence surrounding the asparagine determines its susceptibility to glycosylation.
- The consensus sequence for N-linked glycosylation is Asn-X-Ser/Thr, where X can be any amino acid except proline.
Synthesis
The intermediate compound of the P-P-dolichol-linked glycosidic bond plays a crucial role in the synthesis of N-linked glycoproteins. Initially, sugars are attached to the dolichol pyrophosphoryl skeleton. The protein core is synthesized on ribosomes attached to the rough endoplasmic reticulum. The sugar chain linked to dolichol is then transferred to the asparagine residues present in the protein.
Biological Significance
Lectins: These are proteins that bind to specific sugars. Some lectins are glycoproteins themselves. Lectins play a role in cell-to-cell adhesion. Each lectin has two sugar-binding sites. Lectins with only one sugar-binding site cannot connect cells to each other. The asialoglycoprotein receptor is an example of a human lectin.
Selectins: These are a group of lectins found on the surface of leukocytes and endothelial cells. Selectins are membrane-bound proteins that bind to calcium. Leukocytes play an important role in inflammatory and immune processes. Leukocytes often attempt to exit the bloodstream and enter interstitial spaces between cells. The leukocyte exits from the blood and enters into the interstitial space, which is called diapedesis. During diapedesis, selectins bind to specific sugar chains on the surface of neutrophils. The binding of selectins to sugar chains on the surface of neutrophils causes their movement in the blood. Subsequently, neutrophils change shape through specific chemical processes and adhere to the endothelial surface. In the next step, neutrophils pass through the available space between endothelial cells and enter the interstitial space.
Catabolism
At the end of the sugar chains related to glycoproteins, sialic acid is present. Before sialic acid, there is a galactose root. When glycoproteins pass through the liver, enzymes called neuraminidases separate sialic acids from the ends of sugar chains. Upon detachment, the galactose roots of sialic acid-containing sugar chains are exposed at the ends of the sugar chains. On the surface of liver cells, specific asialoglycoprotein receptors recognize the galactose residues in glycoproteins. Through the process of phagocytosis, these receptors pull the glycoproteins into liver cells for degradation.
Conclusion
Glycoproteins are molecules that consist of proteins and carbohydrates linked together. They are found in all living cells and play various roles in biological processes, such as cell signaling, cell recognition, cell adhesion, immune response, and protein folding. Glycoproteins are formed by glycosylation, which is the attachment of sugars to proteins, either during or after protein synthesis.
Glycosylation can affect the structure, stability, activity, and interactions of the protein, as well as its localization and transport within and outside the cell. Glycosylation can also serve as a way of modifying the protein function in response to environmental changes or signals. Glycoproteins are a type of glycoconjugates, which are molecules that have carbohydrates attached to other molecules, such as lipids or nucleic acids.
Reference:
- Chemical Biology of Glycoproteins, edited by Zhongping Tan and Lai-Xi Wang.
- Structural Biology of Glycoproteins, by Joanne E. Nettleship