Glycolysis can be divided into two phases: the preparatory phase and the payoff phase. During the preparatory phase, glucose is converted into two molecules of glyceraldehyde-3-phosphate, consuming two molecules of ATP for each glucose molecule. During the payoff phase, two molecules of glyceraldehyde-3-phosphate are oxidized to form two molecules of pyruvate, resulting in the production of four molecules of ATP.
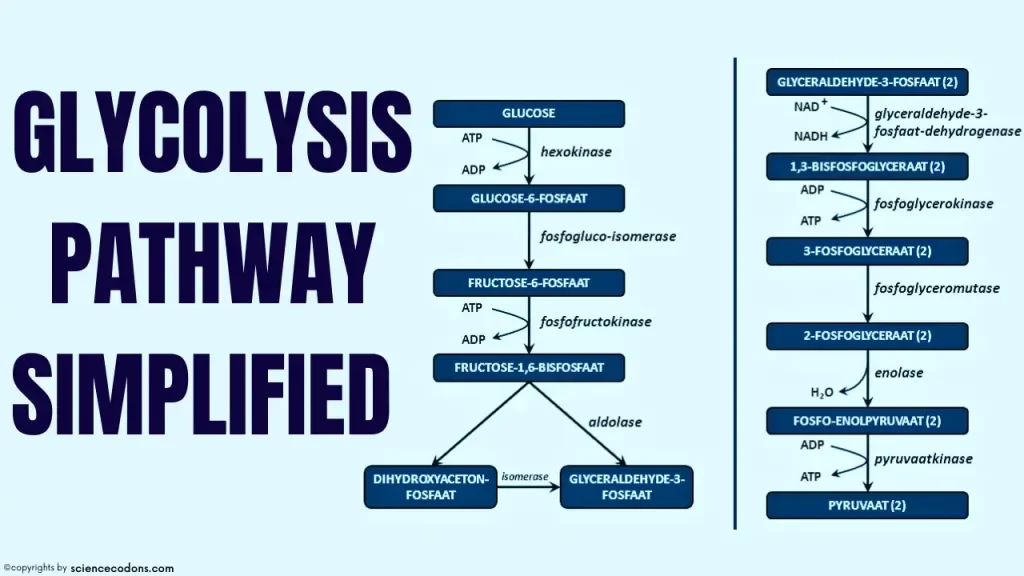
Here is a table that summarizes all the steps of glycolysis, along with the enzymes, substrates, products, and energy yield.
| Step | Enzyme | Substrate | Product | Energy |
|---|---|---|---|---|
| 1 | Hexokinase | Glucose + ATP | Glucose-6-phosphate + ADP | -1 ATP |
| 2 | Phosphoglucose isomerase | Glucose-6-phosphate | Fructose-6-phosphate | 0 |
| 3 | Phosphofructokinase | Fructose-6-phosphate + ATP | Fructose-1,6-bisphosphate + ADP | -1 ATP |
| 4 | Aldolase | Fructose-1,6-bisphosphate | Dihydroxyacetone phosphate (DHAP) + Glyceraldehyde-3-phosphate (G3P) | 0 |
| 5 | Triose phosphate isomerase | DHAP | G3P | 0 |
| 6 | Glyceraldehyde-3-phosphate dehydrogenase | G3P + NAD+ + Pi | 1,3-bisphosphoglycerate (1,3-BPG) + NADH + H+ | +1 NADH |
| 7 | Phosphoglycerate kinase | 1,3-BPG + ADP | 3-phosphoglycerate (3-PG) + ATP | +1 ATP |
| 8 | Phosphoglycerate mutase | 3-PG | 2-phosphoglycerate (2-PG) | 0 |
| 9 | Enolase | 2-PG | Phosphoenolpyruvate (PEP) + H2O | 0 |
| 10 | Pyruvate kinase | PEP + ADP | Pyruvate + ATP | +1 ATP |
1- conversation Glucose to Glucose-6-phosphate (Hexokinase enzyme)
Phosphorylation of glucose to glucose-6-phosphate occurs at carbon number six of glucose and is catalyzed by the enzyme hexokinase. Hexokinase requires magnesium ions for its activity. This reaction is irreversible under cellular conditions. In liver cells, the enzyme called glucokinase is present. This enzyme phosphorylates glucose to glucose-6-phosphate on carbon six using one ATP molecule. Unlike hexokinase, which is present in all body cells, glucokinase is only found in liver cells and pancreatic islets. There are significant differences between glucokinase and hexokinase.

2- conversation Glucose-6-P to Fructose-6-P (Isomerase enzyme)
The enzyme phosphohexose isomerase (or phosphoglucose isomerase) catalyzes the conversion of glucose-6-phosphate to fructose-6-phosphate in a reversible reaction. This enzyme specifically targets the alpha anomer of D-glucopyranose as a substrate and doesn’t affect the beta anomer. The presence of magnesium ions is essential for this reaction. In this reaction, aldose-ketose isomerization occurs, meaning that one aldose (glucose) is isomerized to a ketone sugar (fructose)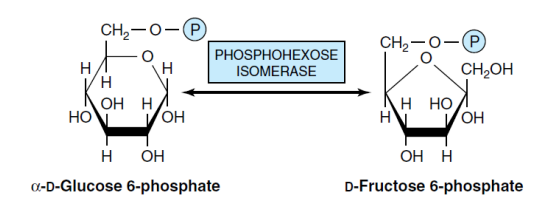
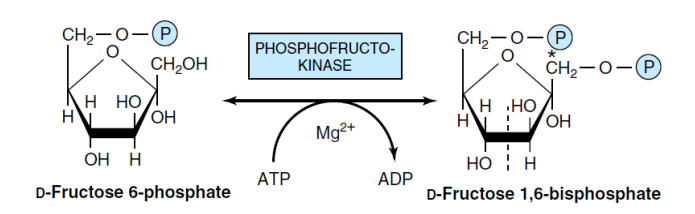
3- conversation Fructose-6-P to Fructose-1,6- bisphosphate (Phosphofructokinase)
Phosphofructokinase-1 (1-PFK) is an enzyme that converts fructose-6-phosphate to fructose-1,6-bisphosphate by utilizing one ATP molecule. This reaction is irreversible under cellular conditions. 1-PFK is a regulatory and main limiting enzyme in the glycolysis pathway. In certain bacteria and plants, a type of 1-PFK enzyme utilizes pyrophosphate (PPi) to phosphorylate fructose.
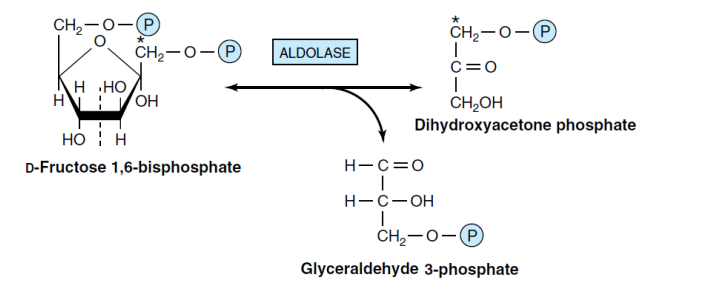
4- breakdown Fructose-1,6-bisP to DHAP+GA3P(Aldolase)
The aldolase enzyme catalyzes the reversible breakdown of fructose-1,6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. Aldolase forms an open chain during the reaction. Aldolase and phosphofructokinase enzymes specifically react with linear fructose and do not react with cyclic fructose.
Aldolase enzymes
There are two types of aldolase enzymes: A and B. Aldolase A is present in most tissues and plays a role in the glycolysis pathway. In contrast, aldolase B is only found in the liver and kidneys and is involved in fructose metabolism. Aldolase in mammals does not require a divalent cation, while the microbial type of this enzyme relies on a divalent cation for activity. Only glyceraldehyde-3-phosphate can sustain glycolysis reactions. The Triosephosphate isomerase enzyme catalyzes the reversible isomerization of dihydroxyacetone phosphate to glyceraldehyde-3-phosphate.
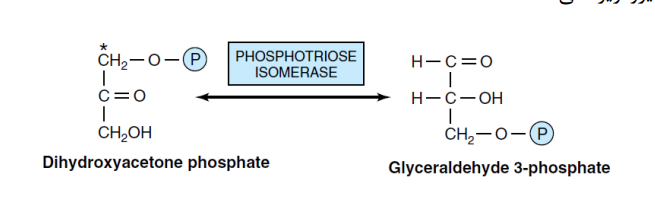
5- conversation DHAP to GA3P (Triose phosphate isomerase)
Isomerization of dihydroxyacetone phosphate to glyceraldehyde-3-phosphate is the final step in phase I of glycolysis. Only glyceraldehyde-3-phosphate is capable of continuing the glycolysis reactions. Therefore, the phosphotriose isomerase enzyme isomerizes dihydroxyacetone phosphate to glyceraldehyde-3-phosphate in a reversible reaction.
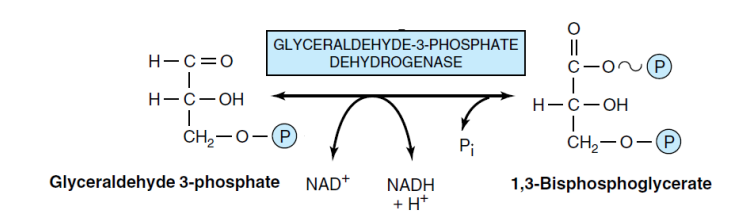
6- Phosphorylation of GA3P to 1,3-bisphosphoglycerate (dehydrogenase enzyme)
Phosphorylation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate is catalyzed by the enzyme glyceraldehyde-3-phosphate dehydrogenase, using inorganic phosphate to add a phosphate group to carbon number one of glyceraldehyde-3-phosphate. The enzyme’s active site contains a thiol group (SH-) derived from the amino acid cysteine.
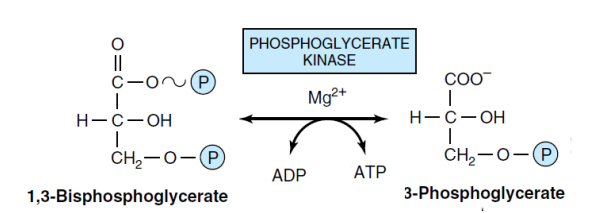
7- conversation 1,3-bis-PG to 3-PG (Kinase)
Phosphoglycerate kinase enzyme converts 1,3-bisphosphoglycerate to 3-phosphoglycerate. In this reaction, one molecule of ATP is produced. This type of ATP production is called substrate-level phosphorylation.
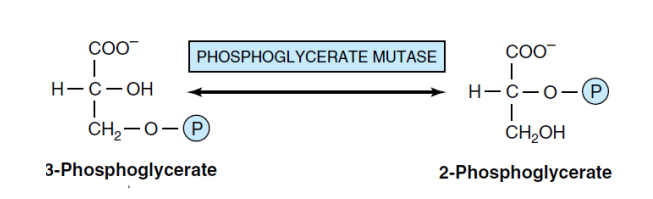
8- conversation 3-PG to 2-PG (mutase)
Phosphoglycerate mutase is an enzyme that catalyzes the conversion of 3-phosphoglycerate to 2-phosphoglycerate. The presence of magnesium ions is essential for this reaction. In converting 3-phosphoglycerate to 2-phosphoglycerate, the compound 2,3-bisphosphoglycerate acts as an intermediate. The mutase enzyme has a histidine in its active site bound to a phosphate. Initially, the enzyme attaches a phosphate to carbon 2 on 3-phosphoglycerate, producing 3,2-bisphosphoglycerate. Then, the enzyme removes the phosphate from carbon three using its histidine residue, producing the final product of the reaction, 2-phosphoglycerate.
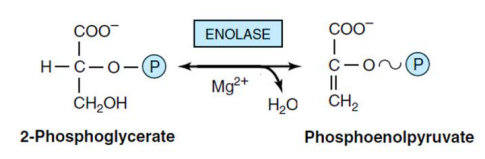
9- conversation 2-PG toPEP (Enolase enzyme)
An enzyme removes a water molecule from 2-phosphoglycerate and converts it to phosphoenolpyruvate. This reaction is reversible under intracellular conditions. The removal of a water molecule causes energy redistribution in the molecule. This enzyme requires magnesium and manganese ions for activity.
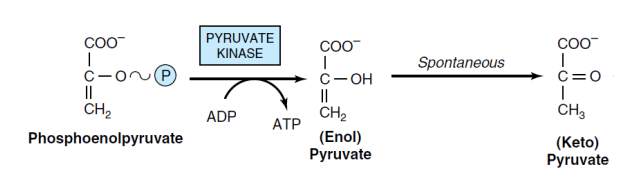
10- the final step of Glycolysis pathway (pyruvate kinase)
The enzyme pyruvate kinase catalyzes the reversible conversion of phosphoenolpyruvate to pyruvate. The reaction produces enol pyruvate, which spontaneously and non-enzymatically converts to keto pyruvate. In this reaction, phosphorylation occurs at the substrate level, producing one molecule of ATP. Potassium, manganese, or magnesium ions are necessary for this reaction.