Both myoglobin and hemoglobin are essential proteins responsible for binding and transporting oxygen throughout the body. However, despite their shared function, they differ in crucial ways, like structure, location, and purpose. Let’s delve into the intriguing world of these fascinating molecules.
| Feature | Myoglobin | Hemoglobin |
|---|---|---|
| Structure | Monomer | Tetramer |
| Number of subunits | 1 | 4 |
| Location | Muscle tissue | Red blood cells |
| Function | Stores oxygen in muscle cells | Transports oxygen from lungs to tissues |
| Protein part | Single chain with 153 amino acids | Four polypeptide chains |
| Heme part | One heme group | Four heme groups |
| Oxygen binding affinity | Higher | Lower |
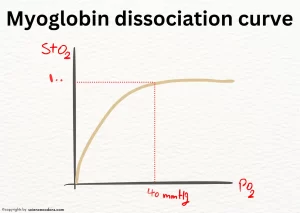
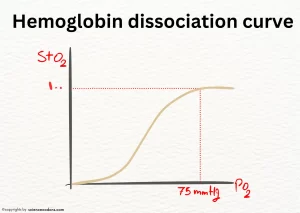
| Curve in graph of oxygen dissociation | Hyperbolic | Sigmoid |
| Molecular weight | 16.7 kDa | 64 kDa |
| Abbreviation | Mb | Hb |
Compare myoglobin & hemoglobin in structure
Hemoglobin and myoglobin are composed of two parts (protein and heme). The protein part in myoglobin is a monomer, a single-chain protein (made up of 7 alpha-helical pieces) with 153 amino acids. While the protein part in hemoglobin consists of four polypeptide chains(heterotetramer). Both heme parts are similar in these two proteins. They are both protoporphyrin and are responsible for binding to oxygen.
Deep in the structure of myoglobin & hemoglobin
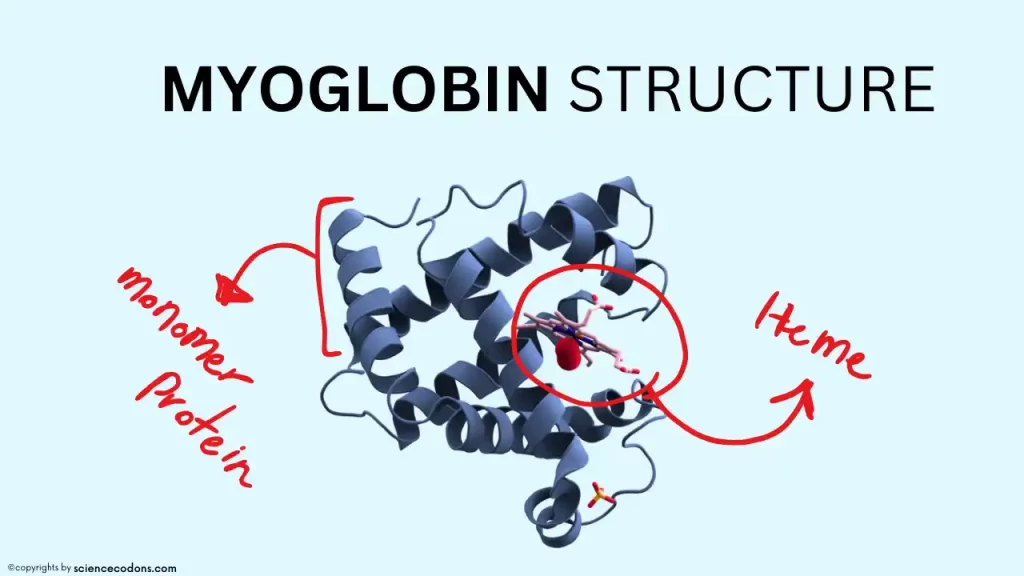
Myoglobin(17000 daltons) is a hemoprotein consisting of two parts: a heme part and a protein part of 153 amino acids long. Approximately 70% of the secondary structure of myoglobin is composed of alpha-helices. Myoglobin has eight alpha-helical structures named A through H in order. Heme is located between helices E and F in myoglobin. Two histidine roots (proximal histidine or F8 and distal histidine or E7) interact in the protein part. Unlike other amino acids in the inner part of myoglobin, which are nonpolar, these histidine roots are polar. Four iron capacities are occupied by nitrogen atoms from pyrrole rings. Histidine F8 occupies the fifth iron position. Histidine E7 plays a role in protecting them.
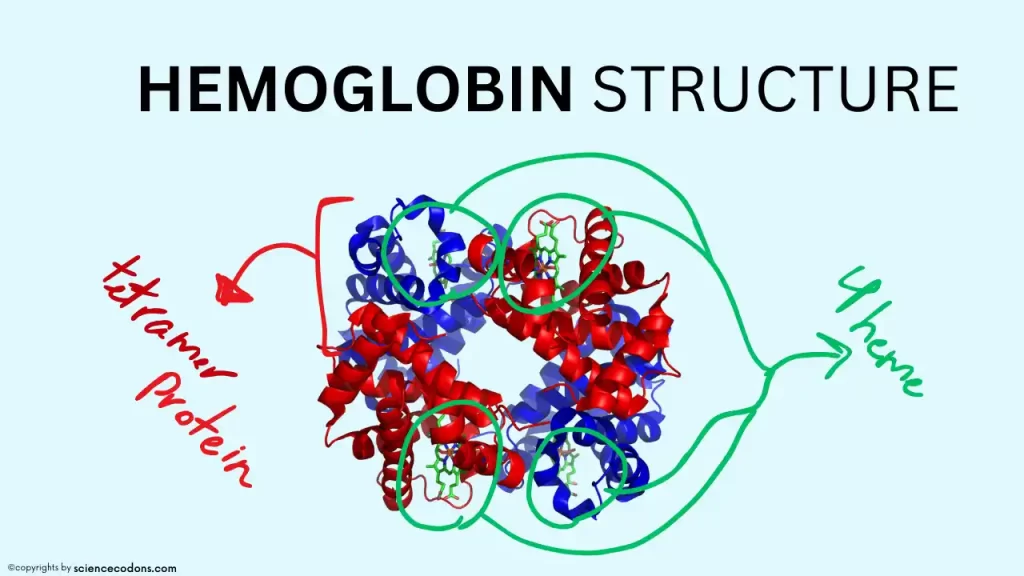
Hemoglobin is a tetramer protein that consists of four polypeptide chains. These chains are similar to each other in pairs. The chains in the hemoglobin structure are different at different stages of life. The amino acid sequence of these chains is entirely different from each other, and therefore, they have different shapes and functions. Some of these chains are synthesized only in one period of life. Gower-I and Gower-II hemoglobins are synthesized only in the first three months of fetal life.
HbF is the primary hemoglobin from the third month of fetal life until the end of fetal life, and its level in the blood decreases rapidly after birth. In an adult, only 0.5% of the hemoglobin in the blood is of the HbF type. After birth, the primary hemoglobin in the blood is HbA, and the minor hemoglobin is HbA2. In an adult, 98% of the hemoglobin in the blood is of the HbA type, and 1% is of the HbA2 type.
Function: myoglobin vs hemoglobin
The function of each protein depends on its three-dimensional structure. Hemoglobin and myoglobin are two examples of proteins that can best understand the relationship between the structure and function of proteins by examining them.
Hemoglobin plays a role in transferring oxygen from the lungs to the tissues, buffering the blood, and transferring carbon dioxide from the tissues to the lungs. But, myoglobin plays a role in storing oxygen in the muscles. Myoglobin stores oxygen in the muscles and releases it when the oxygen pressure in the muscles decreases to be used for energy production.
Hemoglobin and myoglobin dissociation curve
AS myoglobin has one heme, it can only bind to one oxygen molecule. Myoglobin has a very high tendency to bind to oxygen and only releases its oxygen when the oxygen pressure in the cells decreases very much (less than 1mmHg) and makes it available to the cell. Also, myoglobin has very low sensitivity to changes in oxygen pressure inside the cell at pressures above 5mmHg. These two issues are clearly visible in the oxygen binding diagram of myoglobin, which has a hyperbolic shape.
Hemoglobin has four hemes, so it can bind to four oxygen molecules. Hemoglobin bound to oxygen is called oxyhemoglobin (R state) or oxygenated, and hemoglobin without oxygen is called tense (T state) or deoxyhemoglobin. The oxygen binding diagram of Hemoglobin has a Sigmoid shape.
in conclusion
Myoglobin and hemoglobin, though similar in their oxygen-binding role, are distinct players in the grand game of life. Hemoglobin, the mobile transporter, delivers oxygen throughout the body, while myoglobin, the resident storekeeper, keeps muscles fueled for action. Understanding these oxygen chaperones sheds light on the intricate workings of our bodies and the delicate balance that sustains life.
Reference:
Biochemistry, Myoglobin – StatPearls – NCBI Bookshelf (nih.gov)
wikipedia.org/wiki/Hemoglobin