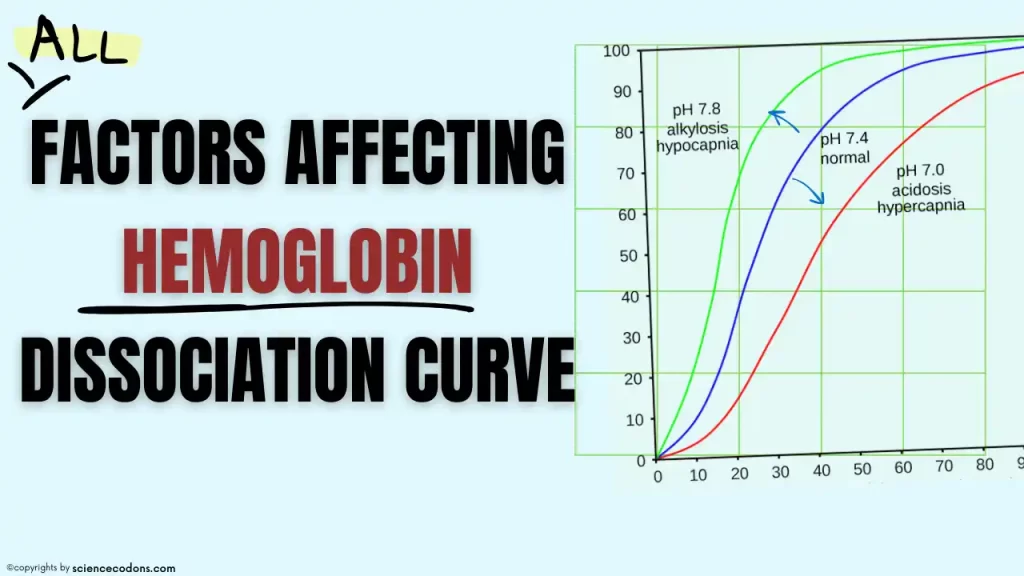
Some factors can affect the affinity of hemoglobin for oxygen(oxygen-hemoglobin dissociation curve). Decreasing pH, increasing protons, increasing carbon dioxide, increasing body temperature, increasing 2,3-bisphosphoglycerate, and increasing potassium ion concentration decreases the affinity of hemoglobin for oxygen. In contrast, increasing pH, increasing the concentration of HbF in the blood, decreasing cold, and decreasing carbon dioxide and 2,3-bisphosphoglycerate increase the affinity of hemoglobin for oxygen.
The change in hemoglobin affinity for oxygen can be understood from the shift of the curve to the left or right. Factors that reduce the affinity of hemoglobin for oxygen shift the curve to the right, and factors that increase the affinity of hemoglobin for oxygen shift the curve to the left.
Let’s talk more…
How does pH affect hemoglobin’s affinity for oxygen?
- Decreased pH (acidity): hemoglobin releases oxygen more easily for tissue use.
- Increase in pH (alkalinity): increasing hemoglobin’s affinity for oxygen.
One of the important processes in the human body is transporting oxygen and carbon dioxide by the blood. First, carbon dioxide (CO2) is produced from the metabolism of different substances in environmental tissues. Next, CO2 is able to diffuse through the cell membrane and enter the blood. Then, CO2 passes through the plasma membrane of red blood cells and enters these cells. After that, inside these cells, the enzyme carbonic anhydrase reacts CO2 with water and produces carbonic acid (H2CO3). Carbonic anhydrase needs zinc for its activity.
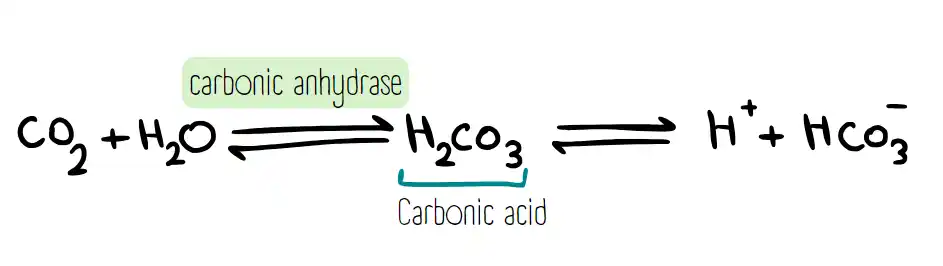
H2CO3 is a weak acid and spontaneously decomposes into bicarbonate ion (HCO3-) and proton (H+). The proton binds to hemoglobin (Hb) and causes the release of oxygen (O2) from Hb. The relationship between pH and O2 release is called the Bohr effect, which states that lower pH (higher acidity) reduces the affinity of Hb for O2, and higher pH (lower acidity) increases the affinity of Hb for O2. Therefore, as a general result, it can be said that the more CO2 is produced in a tissue, the more protons will be produced, and the more O2 will be released from Hb.
What is the effect of 2,3-BPG on oxygen transport?
Hemoglobin is a protein that carries oxygen in the blood and has different forms depending on the developmental stage of the organism. First, 2,3-bisphosphoglycerate (2,3-BPG) is produced in the Rapoport-Luebering shunt in red blood cells. Next, after production, this molecule binds to the space between the two B subunits and causes the conversion of state R to T to increase. This results in the release of oxygen from hemoglobin.
2,3-BPG has a negative charge and is connected by ionic forces to residues such as lysine and histidine that have a positive charge. In fetal hemoglobin (HbF), serine is located instead of basic amino acids in this part, which is a polar and uncharged amino acid. Therefore, the tendency of HbF to bind to 2,3-BPG is low, and this causes an increase in the affinity of HbF for oxygen.
Summary:
| Factor | Effect on hemoglobin affinity for oxygen | Direction of curve shift |
|---|---|---|
| pH | Lower pH (higher acidity) reduces affinity | Right |
| Temperature | Higher temperature reduces affinity | Right |
| Carbon dioxide | Higher carbon dioxide concentration reduces affinity | Right |
| 2,3-bisphosphoglycerate | Higher 2,3-BPG concentration reduces affinity | Right |
| Hemoglobin type | Fetal hemoglobin (HbF) has higher affinity than adult hemoglobin (HbA) | Left |
| Carbon monoxide | Carbon monoxide binds to hemoglobin and reduces its availability for oxygen | Left |