Transthyretin other name
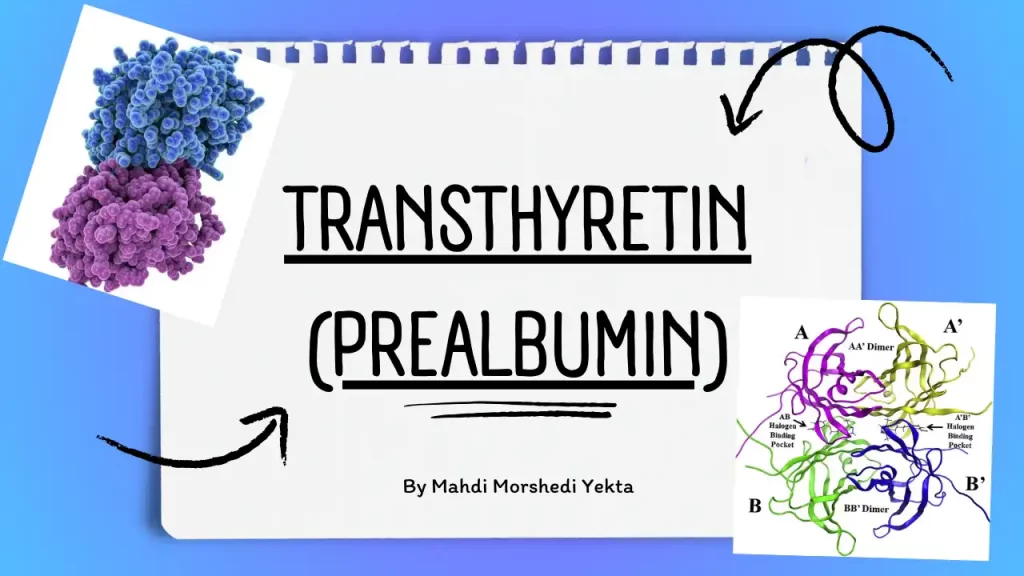
Transthyretin (TTR) or pre-albumin (PA) is a protein with a molecular weight of 35 kDa, devoid of carbohydrates, and composed of four similar polypeptide chains. The term “pre-albumin” was initially used because it migrates faster than albumin in serum protein electrophoresis. However, it was later renamed transthyretin based on its function in binding to thyroid hormones and retinol-binding protein.
Metabolism of Transthyretin
The liver is the primary site of synthesis for pre-albumin (PA), with a smaller amount being produced in the choroid plexus of the central nervous system. The level of PA in the serum is too low to be detected as a separate band in serum protein electrophoresis using conventional techniques. Due to its production in the central nervous system, this protein appears as a distinct band in the electrophoresis of cerebrospinal fluid proteins. The half-life of pre-albumin in circulation is about two days.
What is the role of transthyretin?
Pre-albumin acts as a transport protein in the bloodstream. Approximately 10% of thyroid hormones T4 and T3 are transported in the bloodstream by binding to Pre-albumin (PA), also known as Thyroxine-Binding Pre-albumin (TBPA). T3 has a greater affinity for binding to PA compared to T4.
Pre-albumin also binds to retinol-binding protein (RBP), contributing to vitamin A metabolism. TTR levels are considered a better indicator of nutritional status compared to other proteins like albumin and transferrin due to their short half-life (approximately two days), high tryptophan content, high ratio of essential to non-essential amino acids, and low plasma levels. However, it is crucial to consider the factors contributing to decreased plasma TTR levels, particularly during the acute phase. If TTR levels are used to assess nutritional status, measuring a sensitive acute-phase reactant like CRP alongside TTR is essential. Medical history and physical examination are crucial components of this evaluation.
A variant of transthyretin, in which threonine is replaced by alanine at position 109, is linked to an elevated affinity for thyroxine. This leads to hyperthyroxinemia, characterized by increased thyroxine levels in individuals with normal thyroid function.
Retinol-binding protein (RBP)
Retinol-binding protein (RBP) is a monomeric carrier protein consisting of 182 amino acids and weighing 21 kDa. This protein shares several features with pre-albumin, such as faster movement than albumin and a short half-life of approximately 12 hours. As a result, the level of RBP is also a sensitive indicator of malnutrition.
RBP is synthesized in the liver. RBP synthesis requires retinol to transfer to the Golgi apparatus, which is packaged into secretory granules. Retinol-binding protein (RBP) serves as a carrier for all-trans-retinol, a type of vitamin A, in the bloodstream. Retinol-binding protein (RBP) binds to pre-albumin (PA) in a 1:1 ratio in the choroid plexus. The formation of the PA-RBP complex prevents the renal excretion of RBP, stabilizes retinol binding, and reduces its release to non-target cells.
If the body receives sufficient vitamin A and normal kidney function, it will lead to changes in TTR and RBP levels.
Low levels of transthyretin
Plasma levels of transthyretin are an acute-phase negative reactant and, therefore, decrease in conditions such as malignancy and inflammation. Liver disease and protein-losing disorders (via the kidney or gut) are other causes of reduced blood levels of this protein.
Summary
| Property | Value |
|---|---|
| Structure | Homotetrameric protein is synthesized in the liver, choroid plexus, and retinal pigment epithelium for secretion into the bloodstream, cerebrospinal fluid, and the eye, respectively 123. Each monomer is a 127-residue polypeptide rich in beta-sheet structure 1. The beta-sheet-rich domain forms a dimer of dimers’ quaternary structure |
| Synthesized in | Liver, choroid plexus, and retinal pigment epithelium |
| Quaternary structure | Dimer of dimers |
| Monomer size | 127-residue polypeptide |
| Function | Transports vitamin A (retinol) and thyroxine throughout the body. Transport protein in the plasma and cerebrospinal fluid that carries the thyroid hormone thyroxine (T4) and retinol to the liver |
| Disorder | Transthyretin amyloidosis (ATTR-CM) is a protein disorder that causes clumps of abnormal proteins to build up in your heart and nervous system. It can lead to heart failure, atrial fibrillation, and other complications. |