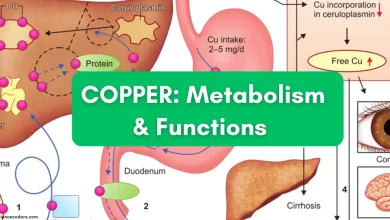
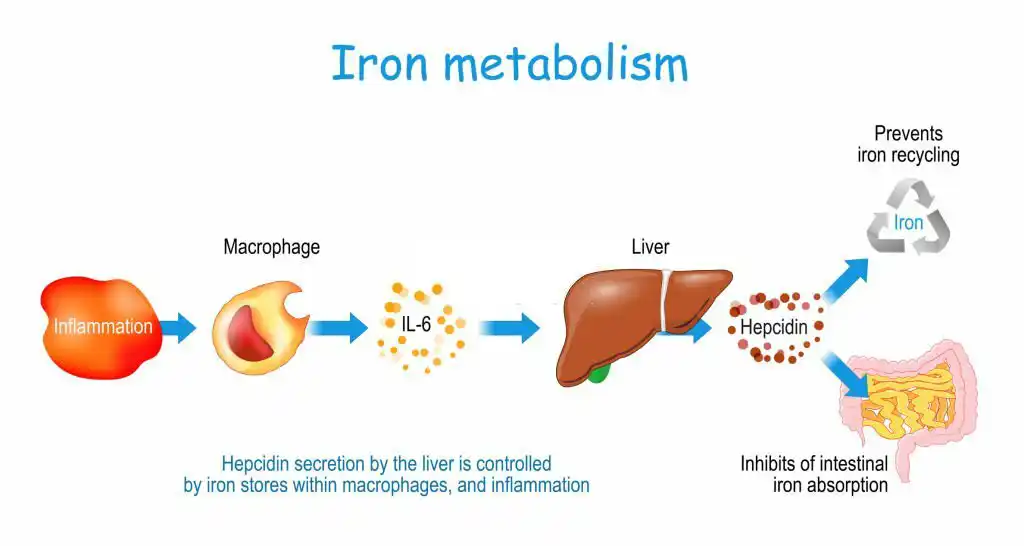
The iron metabolism pathway in the human body is a crucial pathway that involves the absorption, storage, and transfer of iron and plays a critical role in hematopoiesis (the production of blood cells). In this section, we will discuss the three main stages of iron metabolism.
1-Iron Absorption:
Iron absorption begins in the stomach, but mostly occurs in the proximal part of the small intestine. Iron enters the body as ferric ions (+Fe3), which can be bound to ferric oxide or organic solutes that are not absorbable by the cells of the digestive system. The initial step in iron absorption is the conversion of ferric ions to ferrous ions or the reduction of ferric ions. Iron can bind to organic solutes. Three factors affect iron absorption:
- Iron-reducing agents such as vitamin C (ascorbic acid) and sulphur-containing compounds like cysteine convert ferric ions into ferrous ions.
- Vitamin B12, also known as cobalamin, promotes hematopoiesis and increases the body’s iron requirement. It enhances iron absorption.
- Stomach hydrochloric acid and other food acids help to separate iron ions from solutes.
- Iron absorption occurs at a low rate, with only a few milligrams of iron being absorbed per day. When a large amount of iron enters the body, only a small portion is absorbed, and the rest is excreted.
2-Transport of Absorbed Iron
After being absorbed, iron is transported within the body. The liver secretes apo-transferrin protein into the bile, which then enters the second part of the duodenum. The highest amount of iron absorption occurs in this part, although absorption also takes place along the entire length of the small intestine.
Apo-transferrin binds to free iron in the small intestine, as well as iron compounds found in meat foods such as hemoglobin and myoglobin, and then converts into transferrin. Transferrin binds to receptors on the membrane of epithelial cells. The transferring molecule is internalized into the epithelial cell through pinocytosis and later released into the bloodstream as transferrin on the blood side of these cells.
Transferrin binds to receptors on the membrane of immature red blood cells in the bone marrow and enters these cells through endocytosis. Once inside the mitochondria, transferrin is converted into heme molecules.
Storage of Absorbed Iron:
Once inside the body’s cells, ferrous ions attach to a protein called apoferritin and are stored as an iron-containing protein called ferritin. When apoferritin becomes saturated, intestinal absorption of iron ceases. Ferritin is present in the intestinal mucosa, bone marrow, kidney, liver, and spleen.
If the amount of iron in the body is greater than the capacity of apoferritin, some of it is stored in combination with hemosiderin, which is highly insoluble. Unlike ferritin, hemosiderins form large aggregates and are visible in cell staining, while ferritin can only be observed using electron microscopy. When the body needs iron, iron is easily separated from ferritin and goes to other parts of the body by binding to a plasma protein (called apo-transferrin), but it is difficult to separate iron from hemosiderin.
The surplus iron in the bloodstream is deposited in various cells throughout the body, primarily in liver cells and to a lesser extent in reticuloendothelial cells.